Regulatory & Quality
From first submission to final inspection — we make every step confident, compliant, and clear.
Our regulatory and quality teams manage global submissions, ethics coordination, and GCP compliance seamlessly. Whether you’re submitting an IND, CTA, or global dossier, we ensure your documentation is ready for inspection — anywhere, anytime.
We don’t just meet standards — we help define them.
Regulatory & Quality
From first submission to final inspection — we make every step confident, compliant, and clear.
Our regulatory and quality teams manage global submissions, ethics coordination, and GCP compliance seamlessly. Whether you’re submitting an IND, CTA, or global dossier, we ensure your documentation is ready for inspection — anywhere, anytime.
We don’t just meet standards — we help define them.
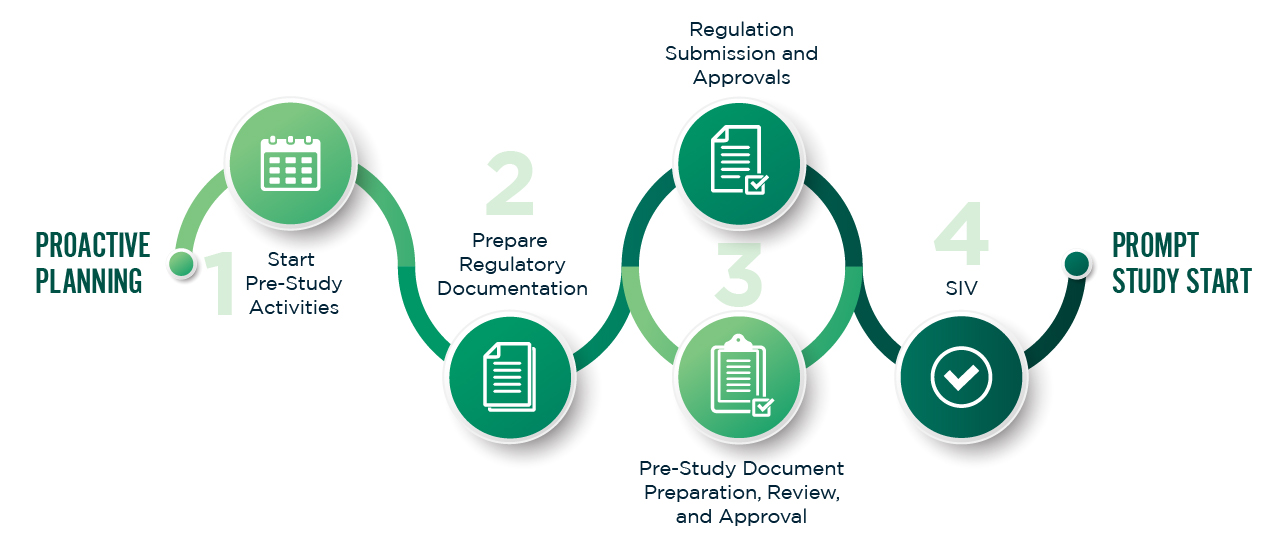

From early consultations to full dossier submission, UNIQ provides strategic regulatory planning for every region.
Our experts prepare CTAs, INDs, and eCTD-ready documentation that meet country-specific requirements — ensuring faster approvals and fewer queries.
We engage proactively with regulators, translating scientific complexity into compliant clarity.
Global experience. Local precision.
Regulatory Strategy & Submissions
Quality Assurance & GCP Compliance
Quality is not a checkpoint. It’s a mindset.
Our Quality Assurance framework integrates risk-based auditing, CAPA oversight, and continuous improvement.
Each project undergoes real-time GCP monitoring, SOP alignment, and pre-inspection readiness checks.
Working With uNIQ: Quality & Compliance You Can Trust
Best Practice, Worldwide
Our Quality Management System is built on globally recognized best-practice standards and fully aligns with ICH-GCP and regional regulatory expectations.
We maintain a structured suite of controlled documents covering quality
manuals, SOPs, policies, and work instructions.
This ensures every study is delivered with consistency, precision, and full compliance — anywhere in the world.
Seamless Integration
Whether your program uses UNIQ's SOPs or sponsor-provided processes, our teams integrate smoothly into your ecosystem. We adapt to your workflows and apply established project-management principles to ensure clarity, alignment, and consistency across every document, form, and study plan. Your operational blueprint remains intact — simply elevated by our execution discipline.
Local Expertise, Wherever You Are
Regulations vary — and we account for every nuance. Our global quality framework incorporates market-specific requirements directly into the QMS, ensuring all submissions, documentation, and site activities meet regional expectations without delay. Local intelligence + global oversight = confident compliance.
Continuous Oversight
Quality at UNIQ is proactive, not reactive. We maintain real-time oversight through:
- Risk-based internal audits
- Vendor assessments
- Continuous data review
- Early identification and resolution of quality issues
The result: inspection-ready operations and complete peace of mind throughout your trial.
Best Practice, Worldwide
Our Quality Management System is built on globally recognized best-practice standards and fully aligns with ICH-GCP and regional regulatory expectations. We maintain a structured suite of controlled documents covering:
- Quality manuals
- SOPs, policies, and work instructions
- Templates, guidelines, and operational procedures
This ensures every study is delivered with consistency, precision, and full compliance — anywhere in the world.
Seamless Integration
Whether your program uses unIQ Trials SOPs or sponsor-provided processes, our teams integrate smoothly into your ecosystem. We adapt to your workflows and apply established project-management principles to ensure clarity, alignment, and consistency across every document, form, and study plan. Your operational blueprint remains intact — simply elevated by our execution discipline.
Local Expertise, Wherever You Are
Regulations vary — and we account for every nuance. Our global quality framework incorporates market-specific requirements directly into the QMS, ensuring all submissions, documentation, and site activities meet regional expectations without delay. Local intelligence + global oversight = confident compliance.
Continuous Oversight
Quality at unIQ Trials is proactive, not reactive. We maintain real-time oversight through:
- Risk-based internal audits
- Vendor assessments
- Continuous data review
- Early identification and resolution of quality issues
The result: inspection-ready operations and complete peace of mind throughout your trial.
